Pyruvate Can Be Metabolized Along Two Major Routes. They Are
The Entner–Doudoroff Pathway
The Entner–Doudoroff pathway is an alternate series of reactions that catabolize glucose to pyruvate.
Learning Objectives
Distinguish between the Entner-Doudoroff pathway and glycolysis
Cardinal Takeaways
Fundamental Points
- Glycolysis is the metabolic pathway that converts glucose into pyruvate and hydrogen ions.
- The Entner-Doudoroff pathway was first reported in 1952 by Michael Doudoroff and Nathan Entner.
- In that location are a few bacteria that substitute classic glycolysis with the Entner-Doudoroff pathway.
Key Terms
- glycolysis: The metabolic pathway that converts glucose into pyruvate and hydrogen ions.
- ATP: Adenosine-five′-triphosphate (ATP) is a multifunctional nucleoside triphosphate used in cells as a coenzyme. Information technology is often called the "molecular unit of currency" of intracellular free energy transfer. ATP transports chemical free energy inside cells for metabolism.

The Entner–Doudoroff Pathway: This is a diagram of the Entner-Doudoroff pathway (KDPG: 2-keto-three-deoxy-6-phosphogluconate).
The Entner–Doudoroff pathway describes an alternate serial of reactions that catabolize glucose to pyruvate using a ready of enzymes different from those used in either glycolysis or the pentose phosphate pathway. Glycolysis (from glycose, an older term for glucose + -lysis deposition) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The gratis free energy released in this process is used to form the loftier-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Most bacteria use glycolysis and the pentose phosphate pathway. This pathway was first reported in 1952 past Michael Doudoroff and Nathan Entner.
Distinct features of the Entner–Doudoroff pathway are that it occurs merely in prokaryotes and information technology uses 6-phosphogluconate dehydratase and 2-keto-3-deoxyphosphogluconate aldolase to create pyruvate from glucose. The Entner–Doudoroff pathway also has a net yield of 1 ATP for every glucose molecule candy, as well as 1 NADH and i NADPH. By comparing, glycolysis has a cyberspace yield of two ATP and ii NADH for every one glucose molecule candy.
There are a few bacteria that substitute classic glycolysis with the Entner-Doudoroff pathway. They may lack enzymes essential for glycolysis, such every bit phosphofructokinase-one. This pathway is generally constitute in Pseudomonas, Rhizobium, Azotobacter, Agrobacterium, and a few other Gram-negative genera. Very few Gram-positive bacteria have this pathway, with Enterococcus faecalis being a rare exception. Virtually organisms that use the pathway are aerobes due to the depression ATP yield per glucose such as Pseudomonas, a genus of Gram-negative bacteria, and Azotobacter, a genus of Gram-negative leaner.
Aerobic Hydrocarbon Oxidation
Microbes tin can utilize hydrocarbons via oxidation as an free energy source.
Learning Objectives
Discuss the advantages of organisms that can undergo aerobic hydrocarbon oxidation
Key Takeaways
Central Points
- Microbes in aerobic atmospheric condition can employ hydrocarbons via oxidation of the hydrocarbon. This leads to byproducts such every bit h2o, alcohol, and peroxide.
- Many hydrocarbons are environmentally damaging, thus the interruption down of hydrocarbons by microbes is of special interest.
- HUM bugs can function as biosurfactants to facilitate the emulsification of hydrocarbons.
Cardinal Terms
- hydrocarbon: A compound consisting simply of carbon and hydrogen atoms.
- biosurfactant: Surface-active substances synthesized by living cells.
- bioremediation: The utilise of biological organisms, unremarkably microorganisms, to remove contaminants, specially from polluted water.
Microbes tin use many different carbon sources for energy. The best known and maybe most common instance is glucose. Microbes tin can utilize hydrocarbons via a stepwise oxidation of a hydrocarbon by oxygen produces water and, successively, an booze, an aldehyde or a ketone, a carboxylic acrid, then a peroxide. Notation the presence of oxygen, thus defining this as aerobic hydrocarbon oxidation. In that location are examples of anaerobic hydrocarbon oxidation, which volition not be discussed here. This is of special involvement as many of the environment pollutants released by human industry are often hydrocarbon based. 1 of the best examples is oil spills. Agreement how microbes assimilate hydrocarbons has started the field of microbial biodegradation, a type of bioremediation. The goal of this is to find ways of using microbes to degrade hydrocarbon spills or waste into less dangerous byproducts such as alcohol.
Hydrocarbon utilizing microorganisms, more often than not Cladosporium resinae and Pseudomonas aeruginosa, colloquially known as "HUM bugs," are commonly present in jet fuel. They live in the h2o-fuel interface of the water droplets, form dark black/brown/greenish, gel-similar mats, and cause microbial corrosion to plastic and rubber parts of the aircraft fuel system by consuming them, and to the metal parts past the means of their acidic metabolic products. They are also incorrectly called algae due to their advent. FSII, which is added to the fuel, acts as a growth retardant for them. At that place are about 250 kinds of bacteria that tin can alive in jet fuel, only fewer than a dozen are meaningfully harmful.

Pseudomonas aeruginosa: P. aeruginosa is capable of growth in diesel fuel and jet fuel, where it is known equally a hydrocarbon-using microorganism (or "HUM bug"), causing microbial corrosion. [3] It creates dark, gellish mats sometimes improperly called "algae" because of their appearance.
Biosurfactants are surface-active substances synthesized by living cells. Interest in microbial surfactants has been steadily increasing in contempo years due to their diversity, environmentally friendly nature, possibility of large-scale production, selectivity, performance under extreme weather condition, and potential applications in environmental protection. Biosurfactants heighten the emulsification of hydrocarbons, have the potential to solubilize hydrocarbon contaminants, and increase their availability for microbial degradation. The use of chemicals for the treatment of a hydrocarbon polluted site may contaminate the environs with their by-products, whereas biological handling may efficiently destroy pollutants, while being biodegradable themselves. Therefore, biosurfactant-producing microorganisms may play an important office in the accelerated bioremediation of hydrocarbon-contaminated sites. These compounds tin can also be used in enhanced oil recovery and may be considered for other potential applications in environmental protection. Other applications include herbicides and pesticides formulations, detergents, healthcare and cosmetics, pulp and paper, coal, textiles, ceramic processing and food industries, uranium ore-processing, and mechanical dewatering of peat. Several microorganisms are known to synthesize surface-agile agents; most of them are bacteria and yeasts. When grown on hydrocarbon substrate as the carbon source, these microorganisms synthesize a broad range of chemicals with surface activity, such as glycolipid, phospholipid, and others. These chemicals are synthesized to emulsify the hydrocarbon substrate and facilitate its transport into the cells. In some bacterial species such as Pseudomonas aeruginosa, biosurfactants are also involved in a group move behavior chosen swarming movement.
The Pentose Phosphate Shunt
The pentose phosphate pathway (PPP) converts glucose-6-phosphate into NADPH and pentoses (5-carbon sugars).
Learning Objectives
Outline the two major phases of the pentose phosphate shunt: oxidative and non-oxidative phases
Key Takeaways
Key Points
- There are two distinct phases in the pathway: the oxidative phase and the non-oxidative phase.
- In the oxidative stage, two molecules of NADP+ are reduced to NADPH, utilizing the energy from the conversion of glucose-6-phosphate into ribulose-5-phosphate. These NADPH molecules can then be used as an energy source in elsewhere in the cell.
- The non-oxidative phase generates v-carbon sugars, which can be used in the synthesis of nucleotides, nucleic acids, and amino acids.
- The pentose phosphate pathway is an alternative to glycolysis.
Key Terms
- glycolysis: The cellular degradation of the elementary saccharide glucose to yield pyruvic acid and ATP as an free energy source.
- NADPH: Nicotinamide adenine dinucleotide phosphate (NADP) conveying electrons and bonded with a hydrogen (H) ion; the reduced course of NADP+.
- oxidative stress: Damage caused to cells or tissue by reactive oxygen species.
The pentose phosphate pathway (PPP; likewise called the phosphogluconate pathway and the hexose monophosphate shunt) is a process that breaks down glucose-vi-phosphate into NADPH and pentoses (5-carbon sugars) for use in downstream biological processes.
At that place are two distinct phases in the pathway: the oxidative stage and the non-oxidative phase. The first is the oxidative phase in which glucose-6-phosphate is converted to ribulose-5-phosphate. During this process 2 molecules of NADP+ are reduced to NADPH. The overall reaction for this process is:

Effigy 1 The Pentose Phosphate Pathway: The pentose phosphate pathway generates reducing equivalents in the class of NADPH. It is used in reductive biosynthesis reactions inside cells (e.g. fatty acid synthesis). Information technology produces ribulose-v-phosphate, used in the synthesis of nucleotides. Information technology also produces nucleic acids and erythrose-4-phosphate, used in the synthesis of aromatic amino acids.
Glucose 6-phosphate + 2 NADP+ + H2O → ribulose-five-phosphate + ii NADPH + 2 H+ + COtwo
The second phase of this pathway is the non-oxidative synthesis of v-carbon sugars. Depending on the body'south state, ribulose-v-phosphate can reversibly isomerize to ribose-five-phosphate. Ribulose-5-phosphate can alternatively undergo a series of isomerizations besides every bit transaldolations and transketolations that outcome in the production of other pentose phosphates including fructose-6-phosphate, erythrose-4-phosphate, and glyceraldehyde-three-phosphate (both intermediates in glycolysis). These compounds are used in a variety of different biological processes including production of nucleotides and nucleic acids (ribose-5-phosphate), as well every bit synthesis of aromatic amino acids (erythrose-four-phosphate).
Glucose-6-phosphate dehydrogenase is the rate-decision-making enzyme in this pathway. It is allosterically stimulated by NADP+. NADPH-utilizing pathways, such as fatty acrid synthesis, generate NADP+, which stimulates glucose-half dozen-phosphate dehydrogenase to produce more NADPH. In mammals, the PPP occurs exclusively in the cytoplasm; it is constitute to be most active in the liver, mammary gland, and adrenal cortex. The ratio of NADPH:NADP+ is commonly almost 100:1 in liver cytosol, making the cytosol a highly-reducing surroundings.
The PPP is one of the 3 main ways the body creates molecules with reducing power, accounting for approximately 60% of NADPH production in humans. While the PPP does involve oxidation of glucose, its primary function is anabolic rather than catabolic, using the energy stored in NADPH to synthesize large, complex molecules from small precursors.
Additionally, NADPH can exist used by cells to prevent oxidative stress. NADPH reduces glutathione via glutathione reductase, which converts reactive HiiOtwo into H2O by glutathione peroxidase. For example, erythrocytes generate a large amount of NADPH through the pentose phosphate pathway to use in the reduction of glutathione.

Fatty Acid Synthesis: Synthesis of Directly-Concatenation Saturated Fat Acids
Organic Acid Metabolism
Microbes tin can harness energy and carbon derived from organic acids past using a variety of dedicated metabolic enzymes.
Learning Objectives
Give examples of types of organic acid metabolism that are used past microorganisms for a sole source of free energy
Key Takeaways
Cardinal Points
- Some microbes are capable of utilizing organic acids such as fatty acids, amino acids, or straight-chain unsaturated acids (e.g., lactate) as a sole source of energy.
- Metabolism of the organic acrid formate is important in methylotrophic organisms. It is vital in the catabolism of C1 compounds (such as methanol).
- Many bacteria are capable of utilizing fatty acids as sole energy and carbon sources through the cyclic β-oxidation pathway, which ultimately yields acetyl-CoA.
Key Terms
- fatty acid: Any of a class of aliphatic carboxylic acids, of general formula CnH2n+1COOH, that occur combined with glycerol as animal or vegetable oils and fats. Just those with an even number of carbon atoms are normally establish in natural fats.
- acyl: Any of grade of organic radicals, RCO-, formed past the removal of a hydroxyl grouping from a carboxylic acrid.
- acetyl CoA: Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production.
Organic Acid Metabolism
A great many organisms generate organic acids (such as lactate) equally a byproduct of fermentation. Some microbes are capable of utilizing such compounds equally a sole source of free energy.
The most commonly metabolized organic acids are the carboxylic acids, which are organic acids containing at least ane carboxyl (-COOH) group. The full general formula of a carboxylic acid is R-COOH, where R is a monovalent functional group. Many types of carboxylic acids can be metabolized by microbes, including:
- Fatty acids (carboxylic acids with long acyl tails)
- Amino acids (the building blocks of proteins)
- Straight-chained, saturated acids (e.g., formate, acetate, and palmitate)
FORMATE METABOLISM
Formate metabolism is important in methylotrophic organisms. It is vital in the catabolism of Cone compounds such equally methanol (see the "Methylotrophy and Methanotrophy" cantlet for more information on Cane chemical compound utilization). Methylotrophic microbes convert unmarried-carbon compounds to formaldehyde, which is oxidized to formate by formaldehyde dehydrogenase. Degradation of formate is then catalyzed past formate dehydrogenase (FDH), which oxidizes formate to ultimately yield COtwo. It permits the donation of electrons to a second substrate (such as NAD+) in the process. This is a critical late step in the hydrocarbon utilization pathway. The ability to metabolize formate is also critical in bacterial anaerobic metabolism, in which example formate is also oxidized by an FDH enzyme but the electrons are donated to cytochromes (proteins involved in electron transport).
FATTY ACID METABOLISM
Many bacteria are capable of utilizing fat acids of diverse tail lengths every bit sole energy and carbon sources. This process requires the β-oxidation pathway, a cyclic process that catalyzes the sequential shortening of fatty acid acyl chains to the final product, acetyl-CoA. The step-by-step process occurs as follows:
- Fatty acid chains are converted to enoyl-CoA (catalyzed by acyl-CoA dehydrogenase).
- Enoyl-CoA is converted to three-hydroxyacyl-CoA (catalyzed by enoyl-CoA hydratase).
- three-hydroxyacyl-CoA is converted to 3-ketoacyl-CoA (catalyzed by 3-hydroxyacyl-CoA dehydrogenase).
- 3-ketoacyl-CoA is thiolated (past 3-ketoacyl-CoA thiolase) to yield one molecule of acetyl-CoA and a derivative of the original input fatty acid that is now shorter by two carbons.
The fatty acid chain that is left over later on the thiolation step tin can then reenter the β-oxidation pathway, which can wheel until the fatty acid has been completely reduced to acetyl-CoA. Acertyl-CoA is the entry molecule for the TCA cycle. The TCA cycle is the procedure used by all aerobic organisms to generate energy.

β-oxidation of fatty acids: Complimentary fatty acids are broken downwardly to acetyl-CoA by defended enzymes in the β-oxidation pathway.
Lipid Metabolism
Biological lipids, which are broken down and utilized though β-oxidation, correspond a potent energy source.
Learning Objectives
Outline the procedure of lipid metabolism, specifically beta-oxidation
Key Takeaways
Fundamental Points
- In addition to their role as the primary component of jail cell membranes, lipids tin can exist metabolized for employ as a chief energy source.
- Lipid metabolism involves the degradation of fatty acids, which are fundamental biological molecules and the building blocks of more structurally complex lipids.
- In order to be metabolized by the cell, lipids are hydrolyzed to yield free fatty acids that and so converted to acetyl-CoA through the β- oxidation pathway.
- One major feature of anaerobic digestion is the product of biogas (with the most useful component being methane), which tin can exist used in generators for electricity production and/or in boilers for heating purposes.
Key Terms
- carboxylic acid: Any of a grade of organic compounds containing a carboxyl functional grouping.
- coenzyme A: A coenzyme, formed from pantothenic acrid and adenosine triphosphate, that is necessary for fatty acid synthesis and metabolism.
Lipid Metabolism
Lipids are universal biological molecules. Not only does this broad class of compounds stand for the chief structural component of biological membranes in all organisms, they besides serve a number of vital roles in microorganisms. Among these, lipids tin can be metabolized by microbes for employ every bit a main free energy source. Although non stated explicitly, the "Organic Acrid Metabolism" atom in this module introduces the concept of lipid metabolism by describing the procedure of fatty acid metabolism through β-oxidation. This atom will aggrandize on the metabolic pathway that enables degradation and utilization of lipids. Fatty acids are the edifice blocks of lipids. They are fabricated of a hydrocarbon concatenation of variable length that terminates with a carboxylic acid group (-COOH). The fat acid structure (see below) is one of the nearly primal categories of biological lipids. It is commonly used every bit a edifice block of more structurally complex lipids (such as phospholipids and triglycerides). When metabolized, fatty acids yield large quantities of ATP, which is why these molecules are important energy sources. Lipids are an energy and carbon source. Before complex lipids can be used to produce free energy, they must first exist hydrolyzed. This requires the activeness of hydrolytic enzymes chosen lipases, which release fatty acids from derivatives such as phospholipids. These fat acids tin then enter a dedicated pathway that promotes pace-wise lipid processing that ultimately yields acetyl-CoA, a critical metabolite that conveys carbon atoms to the TCA wheel (aka Krebs cycle or citric acid cycle) to be oxidized for energy production.

An instance of a fat acrid: A fat acid is a carboxylic acid with a long aliphatic tail that may exist either saturated or unsaturated. The molecule shown hither is the eight-carbon saturated fat acid known as octanoic acid (or caprylic acid).
β-oxidation
The metabolic procedure by which fatty acids and their lipidic derivatives are broken down is called β-oxidation. This procedure bears meaning similarity to the mechanism by which fatty acids are synthesized, except in opposite. In brief, the oxidation of lipids proceeds as follows: two-carbon fragments are removed sequentially from the carboxyl end of the fatty acid after dehydrogenation, hydration, and oxidation to form a keto acid, which is then broken by thiolysis. The acetyl-CoA molecule liberated by this procedure is eventually converted into ATP through the TCA cycle.
β-oxidation can exist broken downwards into a series of discrete steps:
- Activation: Before fat acids tin be metabolized, they must exist "activated. " This activation step involves the improver of a coenzyme A (CoA) molecule to the stop of a long-concatenation fat acid, afterwards which the activated fatty acid (fatty acyl -CoA) enters the β-oxidation pathway.
- Oxidation: The initial footstep of β-oxidation is catalyzed by acyl-CoA dehydrogenase, which oxidizes the fatty acyl-CoA molecule to yield enoyl-CoA. As a effect of this process, a trans double bond is introduced into the acyl chain.
- Hydration: In the 2d step, enoyl-CoA hydratase hydrates the double bail introduced in the previous stride, yielding an booze (-C-OH).
- Oxidation: Hydroxyacyl-CoA dehydrogenase oxidizes the alcohol formed in the previous pace to a carbonyl (-C=O).
- Cleavage: A thiolase then cleaves off acetyl-CoA from the oxidized molecule, which as well yields an acyl-CoA that is two carbons shorter than the original molecule that entered the β-oxidation pathway.
This cycle repeats until the fatty acid has been completely reduced to acetyl-CoA, which is fed through the TCA cycle to ultimately yield cellular energy in the form of ATP.

β-oxidation: The sequential steps of the β-oxidation pathway.
Connecting Proteins to Glucose Metabolism
Backlog amino acids are converted into molecules that tin can enter the pathways of glucose catabolism.
Learning Objectives
Describe the part played by proteins in glucose metabolism
Primal Takeaways
Central Points
- Amino acids must exist deaminated before entering whatever of the pathways of glucose catabolism: the amino group is converted to ammonia, which is used by the liver in the synthesis of urea.
- Deaminated amino acids can exist converted into pyruvate, acetyl CoA, or some components of the citric acrid cycle to enter the pathways of glucose catabolism.
- Several amino acids can enter the glucose catabolism pathways at multiple locations.
Fundamental Terms
- catabolism: Destructive metabolism, usually including the release of energy and breakup of materials.
- keto acid: Any carboxylic acid that likewise contains a ketone grouping.
- deamination: The removal of an amino group from a compound.
Metabolic pathways should be idea of as porous; that is, substances enter from other pathways and intermediates leave for other pathways. These pathways are not airtight systems. Many of the substrates, intermediates, and products in a particular pathway are reactants in other pathways. Proteins are a skillful instance of this miracle. They tin be broken down into their constituent amino acids and used at various steps of the pathway of glucose catabolism.
Proteins are hydrolyzed by a multifariousness of enzymes in cells. Almost of the time, the amino acids are recycled into the synthesis of new proteins or are used equally precursors in the synthesis of other important biological molecules, such as hormones, nucleotides, or neurotransmitters. However, if there are backlog amino acids, or if the body is in a state of starvation, some amino acids will be shunted into the pathways of glucose catabolism.
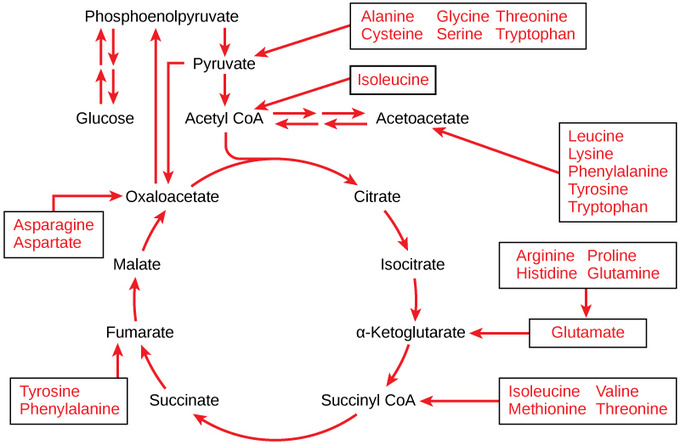
Connection of Amino Acids to Glucose Metabolism Pathways: The carbon skeletons of certain amino acids (indicated in boxes) are derived from proteins and can feed into pyruvate, acetyl CoA, and the citric acrid cycle.
Each amino acid must take its amino group removed (deamination) prior to the carbon concatenation's entry into these pathways. When the amino grouping is removed from an amino acrid, it is converted into ammonia through the urea bike. The remaining atoms of the amino acrid result in a keto acid: a carbon concatenation with one ketone and ane carboxylic acrid group. In mammals, the liver synthesizes urea from two ammonia molecules and a carbon dioxide molecule. Thus, urea is the principal waste matter product in mammals produced from the nitrogen originating in amino acids; it leaves the body in urine. The keto acid can then enter the citric acid bike.
When deaminated, amino acids tin can enter the pathways of glucose metabolism every bit pyruvate, acetyl CoA, or several components of the citric acrid bicycle. For instance, deaminated asparagine and aspartate are converted into oxaloacetate and enter glucose catabolism in the citric acrid bike. Deaminated amino acids can likewise be converted into another intermediate molecule before entering the pathways. Several amino acids can enter glucose catabolism at multiple locations.
Methylotrophy and Methanotrophy
Methylotrophs and methanotrophs are a diverse group of microorganisms that can derive energy from the metabolism of single-carbon compounds.
Learning Objectives
Distinguish between methylotrophs and methanotrophs and their energy sources
Primal Takeaways
Key Points
- Microbes with the ability to use single-carbon (C1) compounds (or multi-carbon compounds lacking carbon bonds) as the sole energy source for their growth are known every bit methylotrophs.
- Methanotrophs, a specific blazon of methylotroph, are able to metabolize methyl hydride as their merely source of carbon and energy.
- Methylotrophs aerobically utilize C1 compounds by oxidizing them to yield formaldehyde, which in turn can either be used for energy or assimilated into biomass.
Central Terms
- methylotroph: Whatever organism that utilizes simple methyl compounds (such as methane or methanol) as a source of carbon and of energy.
- monooxygenase: Any oxygenase enzyme that catalyzes the incorporation of a single atom of molecular oxygen into a substrate, the other atom being reduced to h2o; active in the metabolism of many foreign compounds.
- methanogenesis: The generation of marsh gas by anaerobic leaner.
Methylotrophs
Multiple diverse microorganisms have evolved the intriguing ability to use unmarried-carbon (Ci) compounds (east.g. methanol or methane) or multi-carbon compounds lacking carbon bonds (e.thou. dimethyl ether and dimethylamine) every bit the sole energy source for their growth. Microbes with this adequacy are known as methylotrophs.
Methylotrophs, in general, aerobically use Ci compounds by oxidizing them to yield formaldehyde. Formaldehyde, in turn, tin either be "burned" for energy (by dissimilation to CO2) or assimilated into biomass, assuasive the cell to abound using molecules similar methanol as a sole carbon source. Because methanol is more than arable, more easily purified, and cheaper than carbohydrate carbon sources (eastward.thou. glucose), methylotrophs are particularly useful in biotechnology for the product of amino acids, vitamins, recombinant proteins, single-prison cell proteins, co- enzymes, and cytochromes.

Methylotrophy: This is the general utilization pathway for C1 compounds. Molecules of this blazon are converted to formaldehyde past oxidation and ultimately used as a source of energy and carbon.
Here are examples of methylotrophs:
- Methanosarcina, which tin both apply and produce marsh gas;
- Methylococcus capsulatus, which requires methane to survive; and
- Pichia pastoris, a biotechnologically important model organism that can use methanol as a carbon and energy source.
Methanotrophs
Some methylotrophs can degrade the greenhouse gas marsh gas. Organisms of this type are referred to as methanotrophs. Methanotrophs are able to metabolize methane equally their only source of carbon and energy. About known methanotrophs are bacteria that strictly crave methane for growth ("obligate methanotrophs"). The fact that some methylotrophs can also make utilise of multi-carbon compounds distinguishes them from methanotrophs, which are unremarkably fastidious methane and methanol oxidizers.
Methanotrophs occur more often than not in soils. They are particularly common near environments where marsh gas is produced, such equally:
- oceans
- mud
- marshes
- surreptitious environments
- soils
- rice paddies
- landfills
Methanotrophs are of special interest to researchers studying global warming because they prevent a potential greenhouse gas (marsh gas), far more potent than carbon dioxide, from existence released into the atmosphere. Methanophilic ("methyl hydride-loving") bacteria, therefore, are significant in the global methane budget.

Methylococcus capsulatus: The Methylococcaceae have internal membranes (in the form of flattened discs) that contain pMMOs, which catalyze methane oxidation.
Marsh gas Utilization
Methanotrophs oxidize marsh gas by offset initiating reduction of oxygen (O2) to water (HtwoO) and oxidation of methane (CHiv) to a more active species, methanol (CH3OH), using oxidoreductase enzymes called methane monooxygenases (MMOs). Two types of MMO have been isolated from methanotrophs:
- soluble methyl hydride monooxygenase (sMMO), which is found in the prison cell cytoplasm.
- particulate marsh gas monooxygenase (pMMO), which is establish in the prison cell membrane.
Cells containing pMMO demonstrate higher growth capabilities and college analogousness for methane than cells that contain sMMO. Because pMMO is a membrane protein, cells that use information technology for methane metabolism characteristically have a system of internal membranes within which methyl hydride oxidation occurs.
As in the full general case described above for methylotrophs, methanotrophs ultimately oxidize the methanol produced past MMOs to yield formaldehyde. The method of formaldehyde fixation differs between various methanotrophic organisms. This divergence (along with variability in membrane structure) divides methanotrophs into several subgroups, such as the Methylococcaceae, Methylocystaceae, and Verrucomicrobiae. Although the machinery by which it occurs is not entirely clear, it is as well apparent that sure bacteria can utilize methane anaerobically by substantially running the methanogenesis pathway (normally used by methanogenic bacteria to produce methyl hydride) in contrary. This typically occurs in microbes domicile in marine sediments where oxygen is scarce or altogether absent.
Source: https://courses.lumenlearning.com/boundless-microbiology/chapter/alternatives-to-glycolysis/
0 Response to "Pyruvate Can Be Metabolized Along Two Major Routes. They Are"
Postar um comentário